Specialty Lab Services
and Biomarker Assays:
Driven by Innovation,
Grounded in Science
Precision for Medicine was built specifically to support biomarker-driven development programs, with a strong focus on biomarker assay development.
Our comprehensive approach starts with a foundation of expertise in key biomarker discovery and analysis methods such as immune monitoring, genomics, and bioanalysis. We then specialize in developing new technologies and customized approaches for biomarker assay to generate rich, multi-omic biomarker data from our global footprint. The success of this methodology distinguishes Precision from other contract research organizations and biomarker labs.
Biomarker assays services for any tissue type
We leverage established technologies, proprietary approaches, and develop and validate biomarker assays to generate robust biomarker data in samples ranging from nucleic acids to tissues
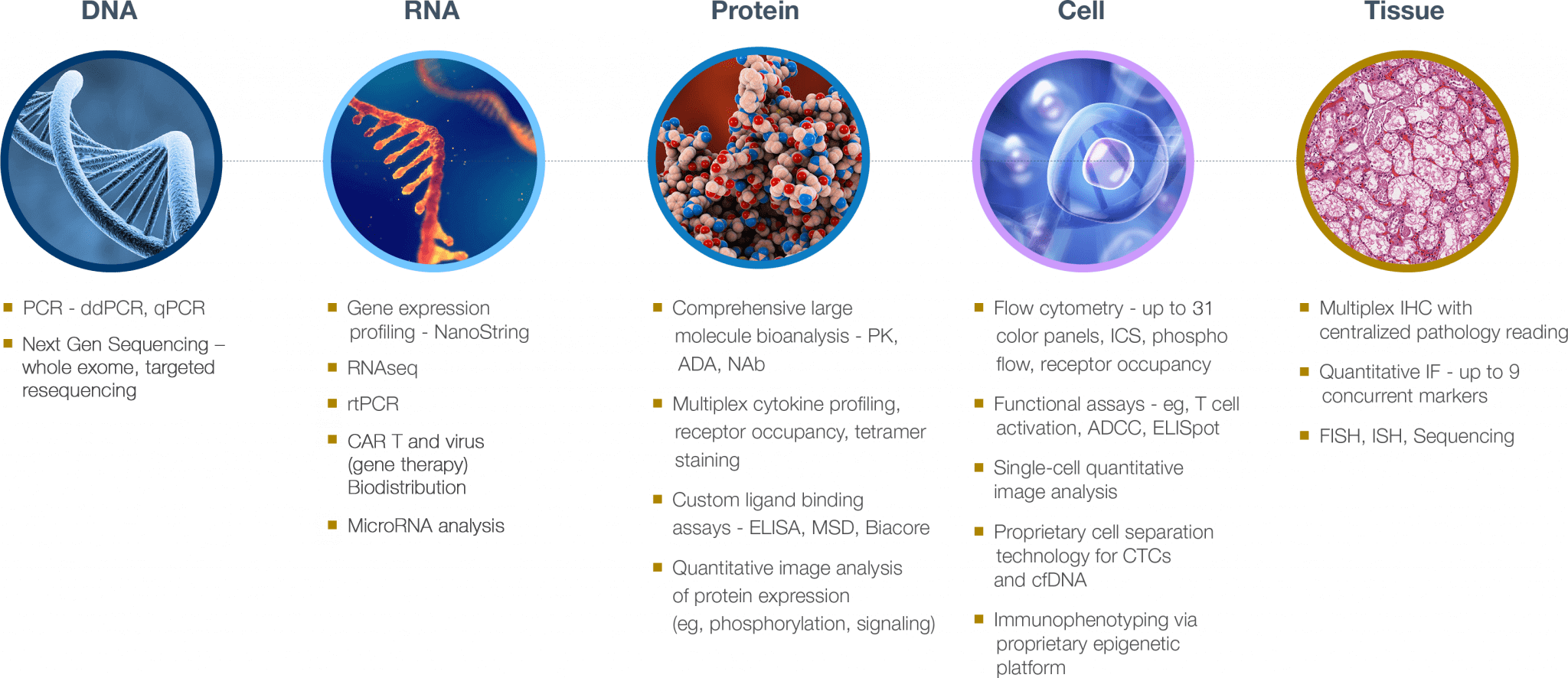

Specialty lab and biomarker assay services
Supporting clinical development programs with a broad range of biomarker platform technologies and approaches
Immune Monitoring
Leverage sophisticated immune monitoring approaches, including flow cytometry, Epiontis ID immune cell phenotyping, and cytokine profiling, to characterize the immune cells in your samples and understand their impact on patient biology.
Flow Cytometry
Acquire detailed insight into cell populations and subpopulations with multiplex flow cytometry, including advanced assays such as tetramer flow, phospho flow, and receptor occupancy measurement.
Genomics
Find and monitor gene expression signatures, copy number variations, chromosome rearrangements, and other genomic events occurring in patient samples using NGS, NanoString, qPCR, ddPCR, and FISH/ISH.
Tissue & Liquid Biopsy
Obtain a detailed molecular view into patient biology with a range of tissue and liquid biopsy technologies, including our proprietary ApoStream platform which can isolate and enrich circulating tumor cells for downstream analysis.
Bioanalytical Testing
Bioanalysis of biologics, cell therapies, gene therapies, and other complex therapeutic types from the globally recognized leaders in immunogenicity testing.
Cytokine Analysis
Build a quantitative understanding of the cytokines present in your sample. We leverage a range of assay technologies to maximize data from even challenging samples.

Translational solutions: Delivering deep science at scale
Globally recognized contract research organization in biomarker-driven development
When you work with Precision you not only gain access to our proprietary technologies, including Epiontis ID for immune cell phenotyping, ApoStream for isolating circulating tumor cells (CTCs), and QuartzBio for integrating, analyzing, and generating insight from diverse clinical and biomarker data, you work directly with the innovators who developed these technologies—scientists who know how to build creative solutions to overcome complex developmental and clinical study challenges.
And while able to execute individual assays as needed, we specialize in holistic solutions that take into account the entirety of a clinical development project, especially for projects where we are working in conjunction with our clinical trials, regulatory, and data science teams for the integrated delivery of solutions for all aspects of a clinical development program.
Expansive global reach ensures rapid and consistent sample processing and analysis
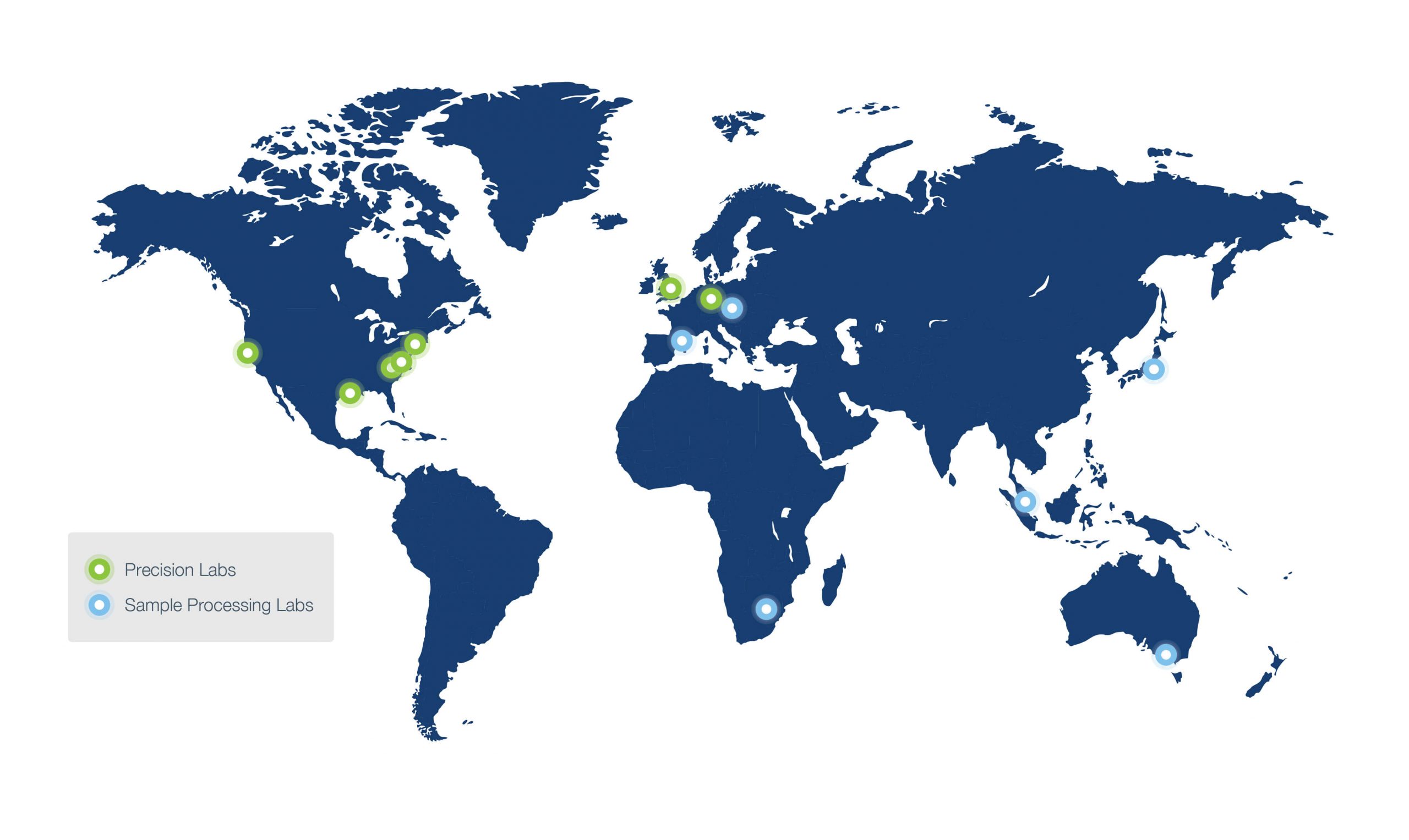
Biospecimens solutions
We stock a range of cryopreserved cells, all of which are ready for immediate domestic and international delivery through our network of distributors. Additionally, we offer prospective collections for any study need.
